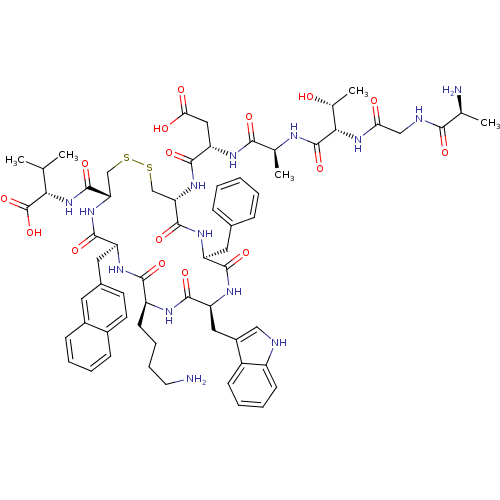
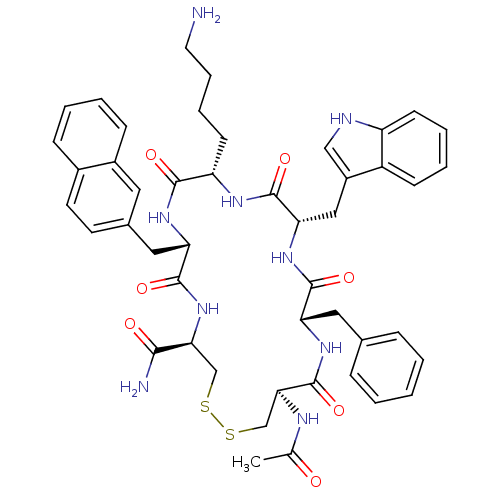
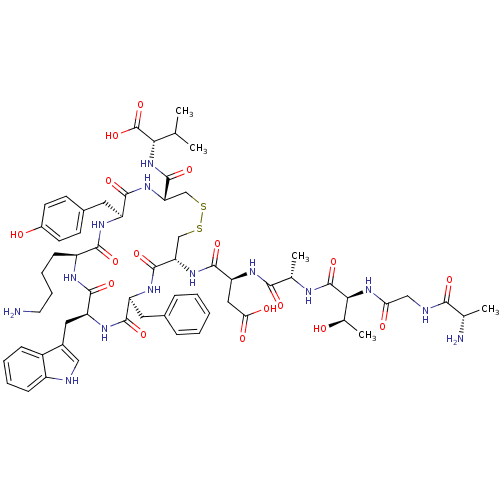
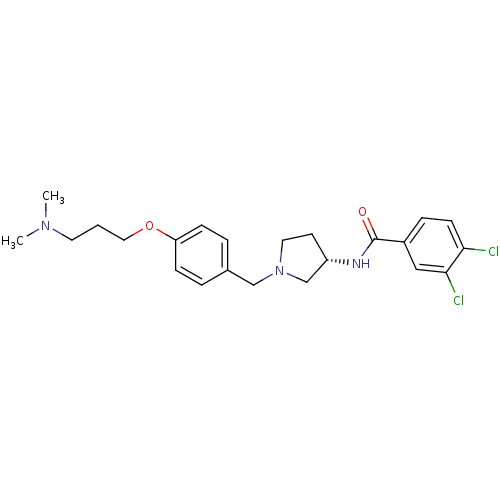
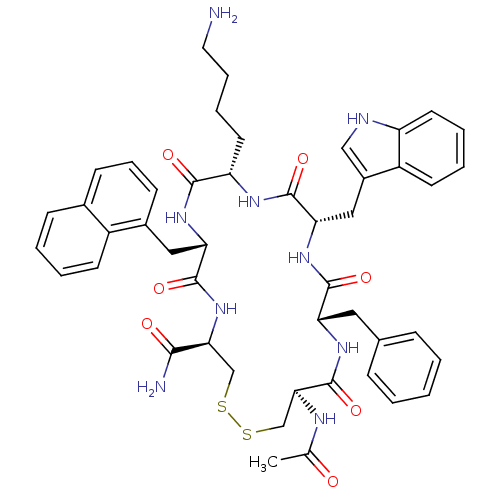
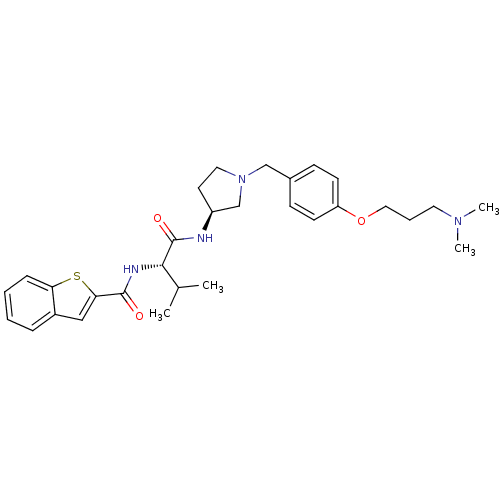
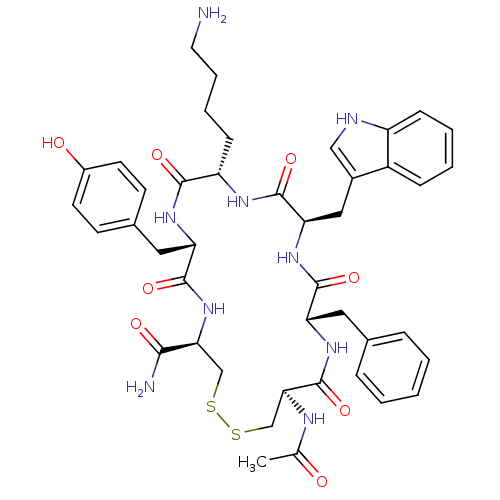
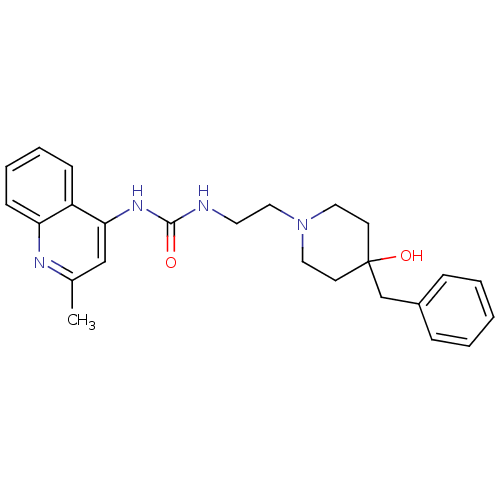
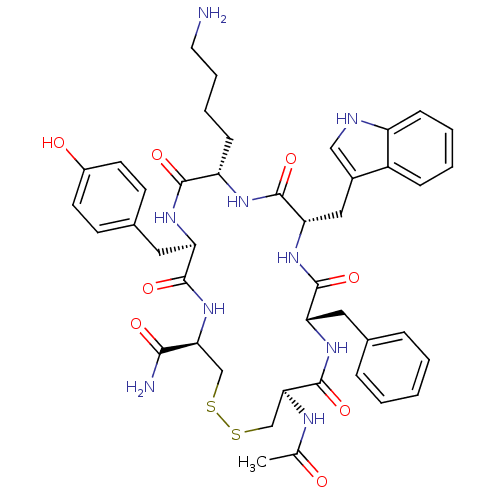
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 0.0200nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
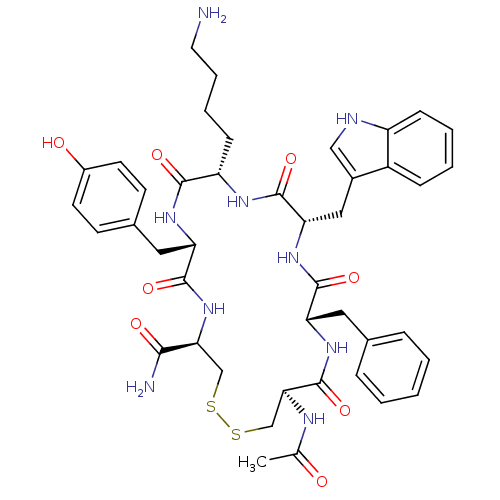
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 0.120nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
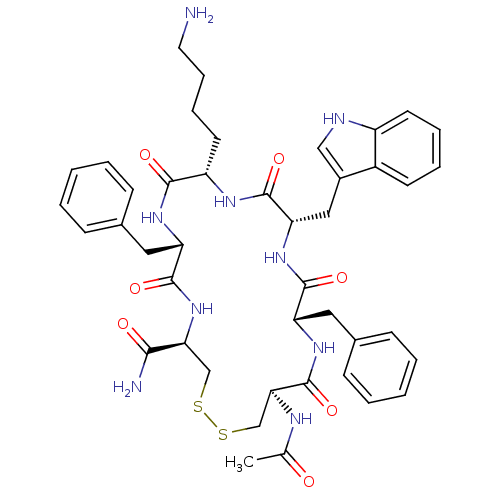
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 0.120nMAssay Description:Displacement of [125I]urotensin 2 from rat urotensin 2 receptor expressed in CHOK1 cells by scintillation proximity assayMore data for this Ligand-Target Pair
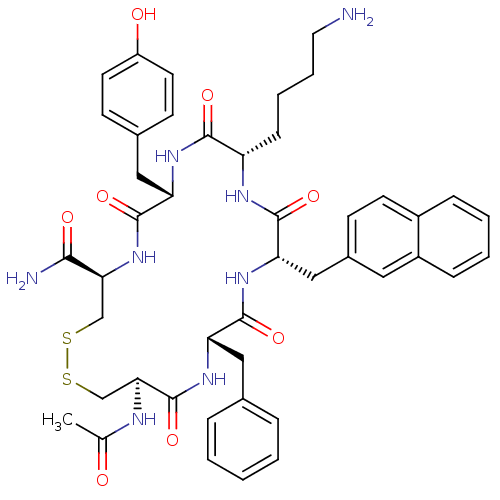
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 0.190nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 4.10nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 6.30nMAssay Description:Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPRMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 34nMAssay Description:Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPRMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 200nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 600nMAssay Description:Agonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization at 1000 nM by FLIPRMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 610nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Antagonist activity at rat urotensin 2 receptor expressed in CHO cells assessed as calcium mobilization by FLIPRMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: 1.10E+3nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
TargetUrotensin-2 receptor(RAT)
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair